Background and Significance
Targeted agents and immunotherapy have changed the treatment landscape for follicular lymphoma (FL), mantle cell lymphoma (MCL), marginal zone lymphoma (MZL), and chronic lymphocytic leukemia (CLL), but indefinite therapy leads to cumulative toxicity and excessive cost. CD47 is a checkpoint for the innate immune system providing an inhibitory signal to phagocytic macrophages and a rational therapeutic target in B-cell lymphoma. Magrolimab is a fully humanized IgG4 anti-human CD47 antibody, with high activity in FL when combined with rituximab (Advani NEJM 2018). Our objective is to test the safety of adding escalating doses of venetoclax to magrolimab and obinutuzumab in a response-adapted study design with planned dose expansion cohorts (www.clinicaltrials.govidentifier NCT04599634).
Study Design and Methods
Patients with grade 1-3A FL, MCL, MZL, or CLL relapsed after and/or refractory to ≥2 prior lines of therapy are eligible. Pts with FL are eligible after 1 prior therapy if FLIPI ≥2 or disease progression within 24 months of the end of the last therapy. Pts with MCL are eligible after 1 prior therapy if blastoid histology, 17p deletion, TP53 mutation, Ki67 ≥30%, or received Bruton's tyrosine kinase inhibitor (BTKi) as first-line therapy. Pts with CLL are eligible after 1 prior therapy if 17p deletion, TP53 mutation, or received both a BTKi and a BCL2 inhibitor. Eligibility includes age ≥18 and adequate organ function unless dysfunction secondary to lymphoma. A positive direct antiglobulin test without hemolytic anemia is allowed. Patients with a history of hemolytic anemia or autoimmune thrombocytopenia within 3 months of enrollment are excluded. No washout period is required for prior anti-lymphoma treatment. Active HIV, CMV, and hepatitis B or C are excluded.
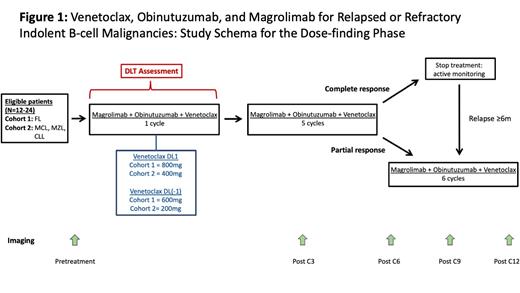
The safety of venetoclax, obinutuzumab, and magrolimab will first be determined in 2 dose-finding cohorts of up to 24 pts (Cohort 1: 6-12 pts with FL and Cohort 2: 6-12 pts with MZL, MCL, or CLL). During dose-finding, all pts start triplet therapy with venetoclax, obinutuzumab, and magrolimab (Figure 1). Magrolimab IV is administered at a 1mg/kg priming dose on D1 of the first cycle, followed by 30mg/kg x 3 weekly loading doses, then 30 mg/kg on D1 of each 4-wk cycle. Obinutuzumab IV is administered at 100mg on D1, 900mg on D2, and 1000mg on D8 and D15 of the first cycle, followed by 1000mg on D1 of each 4-wk cycle. Pts with FL receive venetoclax 800mg (DL1) daily throughout therapy duration, whereas pts with MZL, MCL, and CLL start venetoclax at 20mg with weekly escalation to 400mg (DL1) over 5 weeks. Dose-limiting toxicity is assessed during the first 4 wks for FL, and the first 5 wks for MZL, MCL, and CLL. If ≥2 of 6 pts in either arm experience DLT, 6 additional pts will be enrolled to the respective arm at DL(-1) of venetoclax (600mg for FL and 200mg for MZL, MCL, and CLL).
After dose-finding is completed, expansion cohorts of each histology will first receive magrolimab and obinutuzumab for two 4-wk cycles in a window for translational research. On-treatment tumor biopsies are optional during the window. After the window, venetoclax will be added at the dose determined during dose-finding.
FDG-PET and CT scans are performed at baseline, after window, and after cycles 3 and 6 of triplet therapy. During both dose-finding and dose-escalation, pts with complete response (CR) after 6 cycles of triplet therapy will stop therapy. If these pts relapse, they can be retreated with an additional 6 cycles of triplet therapy. Pts with partial response (PR) after 6 cycles of triplet therapy receive an additional 6 cycles of triplet therapy. All pts stop treatment after 12 cycles. The primary objective is safety, with secondary endpoints of overall response rate, duration of response, and PFS. Exploratory objectives include the identification of a molecular signature that predicts response to magrolimab and obinutuzumab, and correlation of response with circulating tumor DNA levels.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal